Theory and Defination :
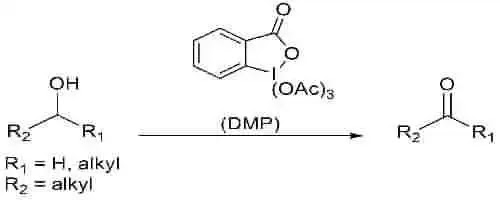
Dess-Martin oxidation is a selective mild oxidation synthesis for the conversion of primary and secondary alcohols into corresponding aldehydes and ketones.The main ingrediant used for this oxidation is Dess-Martin periodinane or DMP.It is named after Daniel Benjamin Dess and James Cullen Martin, two American chemists who invented the periodinane reagent in 1983.Alcohols can be oxidised to aldehydes/ketones under Dess–Martin periodinane conditions without harming furan rings, sulphides, vinyl ethers, or secondary amides. DMP quickly oxidises allylic alcohols, which are normally difficult to convert to their respective carbonyls with conventional oxidants.
General Reaction :
Mechanism :
Firstly DMP prepared ,
And Now DMP used for conversion...
A potential intramolecular elimination of the -hydrogen to produce a C=O bond transforms the periodinane intermediate to the equivalent carbonyl molecule.
Example and Applications :
The Dess–Martin oxidation might be desirable over other oxidation responses as it is exceptionally gentle, keeps away from the utilization of poisonous chromium reagents, doesn't need huge abundance or co-oxidants, and for its simplicity of work up.
1. A gentle general technique for the acyl azides from aldehydes utilizing Dess–Martin periodinane and sodium azide..
The acyl azides are able to be isolated without rearrangement to the alkyl isocyanate due to the mild reaction conditions.ref.link click here
2..
Special Note :-
DMP used to be unavailable commercially because of its explosive potential. Since it is a relatively expensive reagent, one needs to prepare it own.Note that the activity of the reagent tends to vary depending on the amount of residual water during process. DMP and its synthetic precursor (IBX) are hypervalent iodine compounds whose risks as explosives are well known, thus one must plan experimental scale carefully and handle the reagent with necessary caution under supervision.






0 Comments
Post a Comment