Theory and Destination :
The Diels–Alder reaction is an electrocyclic cycloaddition reaction were diene reacts with an alkene (dienophile) in presence of Lewis Acid to give unsaturated six-membered ring.The reaction incorporates [4+2]‑cycloaddition of 4 π-electrons of the framed diene and 2 π-electrons of the dienophile (an alkene or alkyne).This reaction has phenomenal designed importance and was found by two German researchers, Otto Diels and Kurt Alder in 1928. They get the Nobel Prize in 1950.Mechanism :
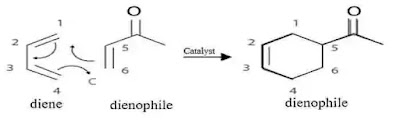
Generally diene - can be either open-chain or cyclic and alkene (dienophile) - electron withdrawing group in conjugation with the alkene , are as shown beloe :
The catalyst used is Lewis acids such as zinc chloride ZnCl2, boron trifluoride BF3, tin tetrachloride SmCl4, or aluminum chloride AlCl3 etc. by binding to the dienophile.
Details mechanism steps are :
🔺Electrons from the dienophile attack carbon C-1 on the diene resulting in a single bond between C-1 and C-6.
🔺Electrons from the double bond between C-1 and C-2 relocates between C-2 and C-3.
🔺Double bond between C-3 and C-4 are broken and the electrons form a single bond between C4 and C5 to form the cyclic product.
🔺All above steps take place in a single step.
Stereochemistry of Diels‐Alder Reaction :
An example of this stereospecificity is the reaction of 1,3‐butadiene with cis‐diethylmaleate.
Retro Diels-Alder Reaction :
retro Diels-Alder reaction is reverse of the Diels-Alder. It passes through the same transition state when the heat is applied. For example, cyclohexene breaks down into butadiene and ethylene at high temperature
Example and Applications :
The first application of Diels–Alder reaction in total synthesis was illustrated by R. B. Woodward's syntheses of the steroids cortisone and cholesterol.
The reaction of butadiene with the quinone gives C and D rings of the steroid syructural derivative with the desired regiochemistry.
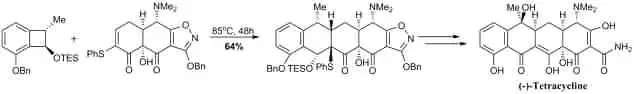
Andrew Myers' 2005 synthesis of (-)-tetracycline[66] achieved the linear tetracyclic core of the antibiotic with a Diels–Alder reaction. Thermally initiated, conrotatory opening of the benzocyclobutene generated the o-quinodimethane, which reacted intermolecularly to give the tetracycline skeleton; the diastereomer shown was then crystallized from methanol after purification by column chromatography. The authors note that the dienophile's free hydroxyl group was integral to the success of the reaction, as hydroxyl-protected variants did not react under several different reaction conditions.
The retro Diels–Alder reaction is used in the industrial production of cyclopentadiene. Cyclopentadiene is a precursor to various norbornenes, which are common monomers. The Diels–Alder reaction is also employed in the production of vitamin.Typical route for production of ethylidene norbornene from cyclopentadiene through vinyl norbornene.







0 Comments
Post a Comment